Groups
LBN team
- Director: Frédéric Cuisinier
- Deputy director:Pierre Yves Collart Dutilleuil
- Second deputy director:Azel Zine
- Secretary: Catherine Barthélémy
- Technical responsible: Fatima Naveen
- Technical assistant: Alban Desoutter
Group 1, Dental Pulp Stem Cells for Dental and Bone Tissue Engineering
Pierre-Yves Collart Dutilleul (Group leader)
- Jacques-Henri TORRES (PU PH)
- Valérie ORTI (PU PH)
- Stéphane BARTHELEMI (PU PH)
- Philippe BOUSQUET (MCU PH)
- Ivan PANAYOTOV (MCU PH)
- Marie-Alix FAUROUX (MCU PH)
- Jean Claude EGEA (MCU-PH)
- Eve MALTHIERY (MCU PH)
- Naveen FATIMA, PhD
- Sophia PIGLIONICO, PhD
TASKS
- Porous silicon for Dental Pulp Stem Cells proliferation and differentiation
- PEEK functionalization for cell adhesion and mineralized tissue formation
- Bifunctional peptide for epithelial adhesion on titanium
- Bone and cartilage formation followed by Raman spectroscopy
- DPSC for anticancer drug delivery
- DPSC Conditioned Medium for cell and tissue regeneration
Group 2, Stem cells, Organoids and Neurosensory Regeneration
Azel ZINE (PU, Group leader)
Groupmembers
- Azel ZINE (PU, Group leader)
- Veronique MONTERO (MCU)
- Olivier ROMIEU (MCU, PH)
- Richard YOUNES (Ph.D Student)
- Yassine MESSAT (Ph.D Student)
- Fidan HUSEYNOVA (Ph.D Student)
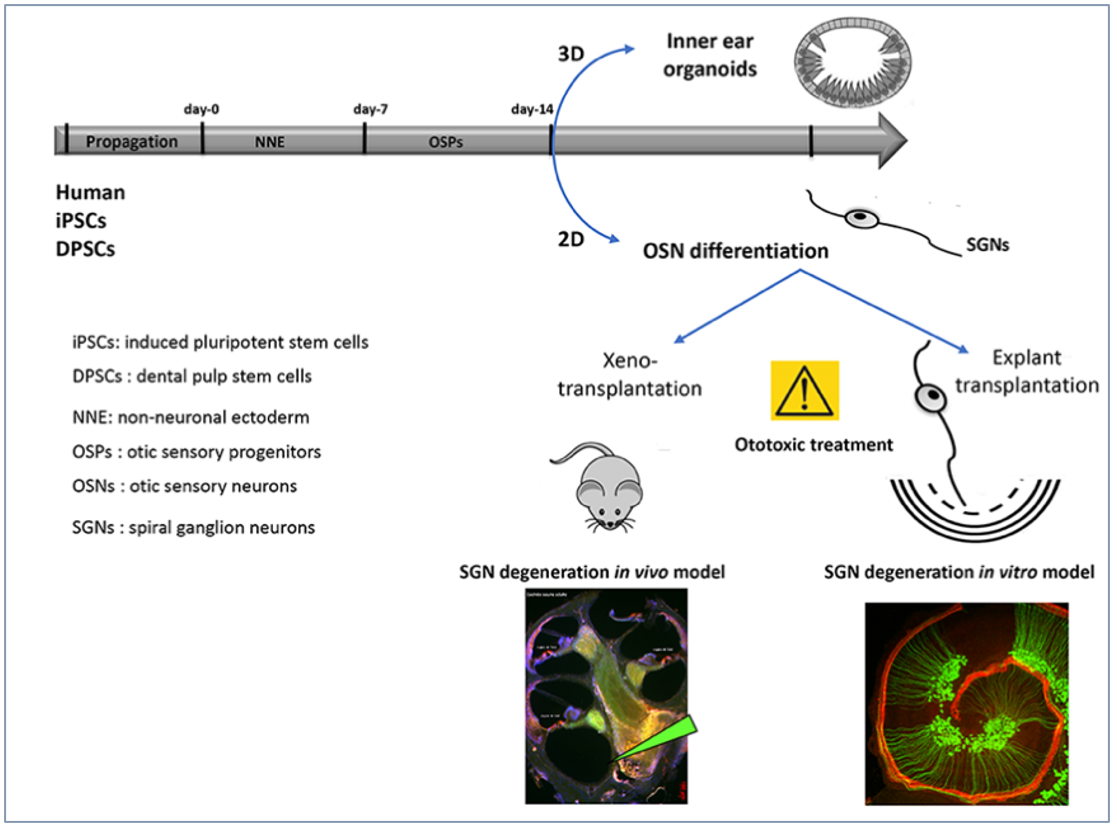
Sensorineural deafness is often caused by the degeneration of spiral ganglion neurons (SGN) in the inner ear that relay auditory signals to the brain. These SGN do not regenerate after degeneration. Dental pulp stem cells (DPSCs) are neural crest-derived ecto-mesenchymal stem cells as the cranial neural crest cells. (NCC) These NCC partially contribute to the SGN during development of the inner ear. DPSCs are promising candidates for cell therapy approach, because they have migratory properties, enabling migration after transplantation can differentiate into sensory neurons and glial cells and can be harvested in relatively high numbers. We hypothesize that human DPSCs can be used for cell-based regeneration and repair of the SGN.
Project 1: Production of human SGN from DPSC and iPSC using 2D and 3D cultures
The main goal of this task is to differentiate and characterize human otic neurosensory progenitors and SGN using a cellular platform for the in vitro production of differentiated cell types from either iPSCs or DPSCs. The stepwise guided differentiation of stem cells into SGN is not straightforward, since there is no single marker which can be applied to distinguish this specific cell type. However, by using RNA-seq high throughput gene expression, it will be possible to more thoroughly determine whether cohorts of known otic neurosensory progenitors and SGN markers over a sequential time course of in vitro differentiation. We will compare a global trajectory towards human otic neurosensory lineage in both DPSCs and iPSCs-derived otic neuronal progenitors and SGN. The development of identity/purity control experiments of otic neuroprogenitors and their neurosensory trajectories will be performed using confocal Raman Spectroscopy in collaboration with Team 3 and Edmos Platform.
Projet 2: Potential of sugar-based angiogenic on organoids vascularization during iPSC and DPSC toward SGN neurosensory differentiation
One of the unique aspects of organoid models is the ability to resemble, to some extent, the in vivo organ or tissue from which they were derived, and which allow for relevant cell-cell and cell-matrix interactions. One of the key limitations in using organoid approaches to generate tissues is that upon reaching a certain size, organoids cease to proliferate and develop a necrotic core. In order to maintain the complexity of organoids, it is necessary to prevent the appearance of the necrotic inner core leading to the premature differentiation in the outer layers of organoids. This phenomenon, in turn, was largely ascribed to a lack of organoid vascularization. We will explore a panel of angiogenic effectors derived from Mannose-6-phosphate (M6P) on the neurodifferentiation of either DPSC or iPSCs, and in the development of organoids vascularization
Project 3: Effects of transplantation neurosensory progenitors in models of SGN degeneration
To test the regeneration and synaptogenesis abilities of DPSC/iPSC-derived otic neurosensory progenitors following engraftment, we will use first anin vitroco-culture model with denervated cochlear organotypic explants. We have previously demonstrated that hiPSCs derived otic progenitors are able to engraft and differentiate in the damaged cochlea (Lopez et al., 2019).
General outlouk of otic neurosesnory differentiation in vitro and engraftement in SGN degeneration models
Collaborations:
- Albert Edge (Harvard Medical School)
- John Devos (IRMB, CHU, Montpellier)
- Christian Chabbert (CNRS, AMU Marseille)
- Pierre Gaudriault (Cherry Biotech, Rennes)
- Annelies Schrott-Fischer (Medical University of Innsbruck, Austria)
Fundings:
Recent publications:
Zine A, Messat Y, Fritzsch B (2021). A human induced pluripotent stem cell-based modular platform to challenge sensorineural hearing loss.Stem Cells. doi: 10.1002/stem.3346.
Kojima K, Nishida AT, Tashiro K, Hirota K, Nishio T, Murata M, Kato N, Kawaguchi S, Zine A, Ito J, Van De Water TR (2020). Isolation and characterization of mammalian otic progenitor cells that can differentiate into both sensory epithelial and neuronal cell lineages.Anat Rec 303(3):451-460.
Lopez A, Lahlou, H, Cazals, Y, Brezun, JM, Ripoll, C, Quan, W, Edge, A, Zine, A (2019). Engraftment of human stem cell-derived otic progenitors in the damaged cochlea.Molecular Therapy27: 1101-1113.
Lahlou H, Nivet E, Lopez A, Fontbonne A, Assou S, Zine A (2018). Enrichment and characterization of human otic sensory progenitors derived from induced pluripotent stem cells (2018).Front Mol Neurosci11:452.
Lahlou, H, Lopez Juarez, A, Fontbonne, A, Nivet, E, Zine, A (2018). Modeling human early otic sensory cell development with induced pluripotent stem cellsPLoS One13(6): e0198954.
Abboud N, Fontbonne A, Watabe I, Tonetto A, Brezun JM, Feron F, Zine A (2017). Culture conditions impact the maturation of traceable, transplantable mouse embryonic stem cell-derived otic progenitor cells.JTissue Eng Regen Med11:2629-2642.
Dinh C, Goncalves S, Bas E, Van De Water T, Zine, A (2015). Molecular regulation of auditory hair cell death and approaches to protect sensory receptor cells and/or stimulate repair following acoustic trauma.Front Cell Neurosci9:96.
Zine A (2014). 3D revolution of stem cells: Generation of ear sensory epithelia in vitro.Med Sci. 30: 952-954.
Zine A, Lowenheim, H, Fritzsch B (2014).Toward translating molecular ear development to generate hair cells from stem cells. In: Kursad T, eds.Adult stem cells.Springer111-161.
Group 3, Photonic biomarkers for teledentistry
Amel Slimani (group leader)
- Hervé Tassery (PU-PH)
- Jean Valcarcel (PU-PH)
- Hamideh Salehi (PR)
- Michel Fages (PU-PH)
- Ivan Panayotov (MCU-PH)
- Jean Cédric Durand (MCU-PH)
- Alban Desoutter (technical assistant LBN)