Group 2
Stem cells, Organoids and Neurosensory Regeneration
Azel ZINE (PU, Group leader)
Group members
- Azel ZINE (PU, Group leader)
- Veronique MONTERO (MCU)
- Olivier ROMIEU (MCU, PH)
- Bayan AASAR (Ph.D Student)
- Meryam ZAROUKI (Technicienne de recherche)
- Maëlis LUCAS (Ingénieur chef de projet)
- Damien VERET (Chercheur-Postdoctorant)
Stem Cells, Organoids and Otic Neurosensory Regeneration
Sensorineural hearing loss is the most frequent human sensory deficit. It is mainly caused by the degeneration of neurosensory cells in the inner ear. The inaccessibility of the human inner ear to surgical biopsy combined with the lack of either human cell or tissue models represents major challenges that has hampered the development of drug and therapies for hearing loss. Addressing these limitations will serve for developing a human cell otic model for oto-protective and oto-regenerative bioassays (Zine et al., Stem Cells 2021).
Project 1
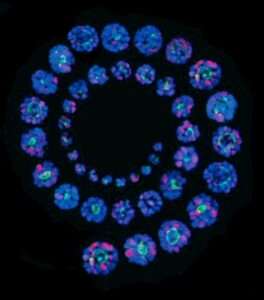
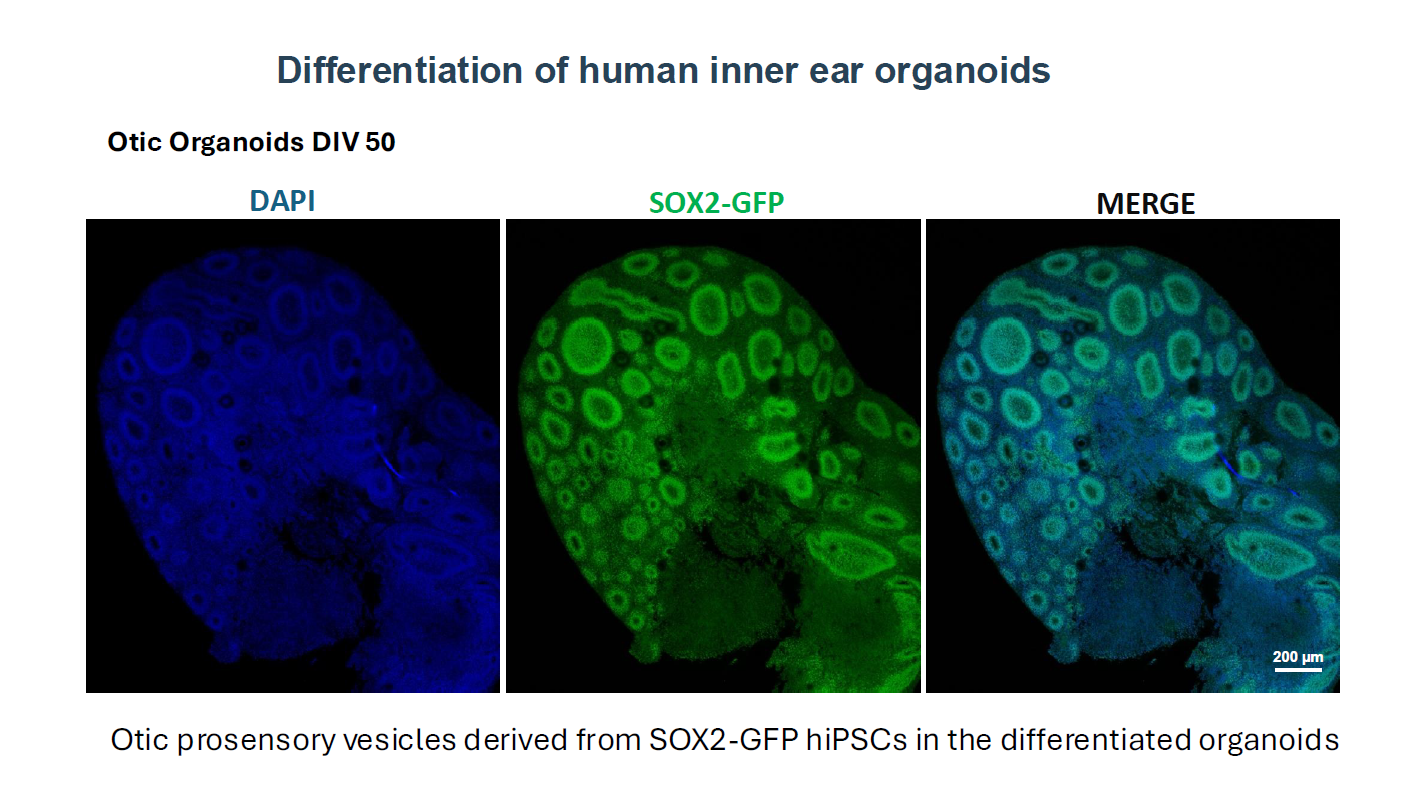
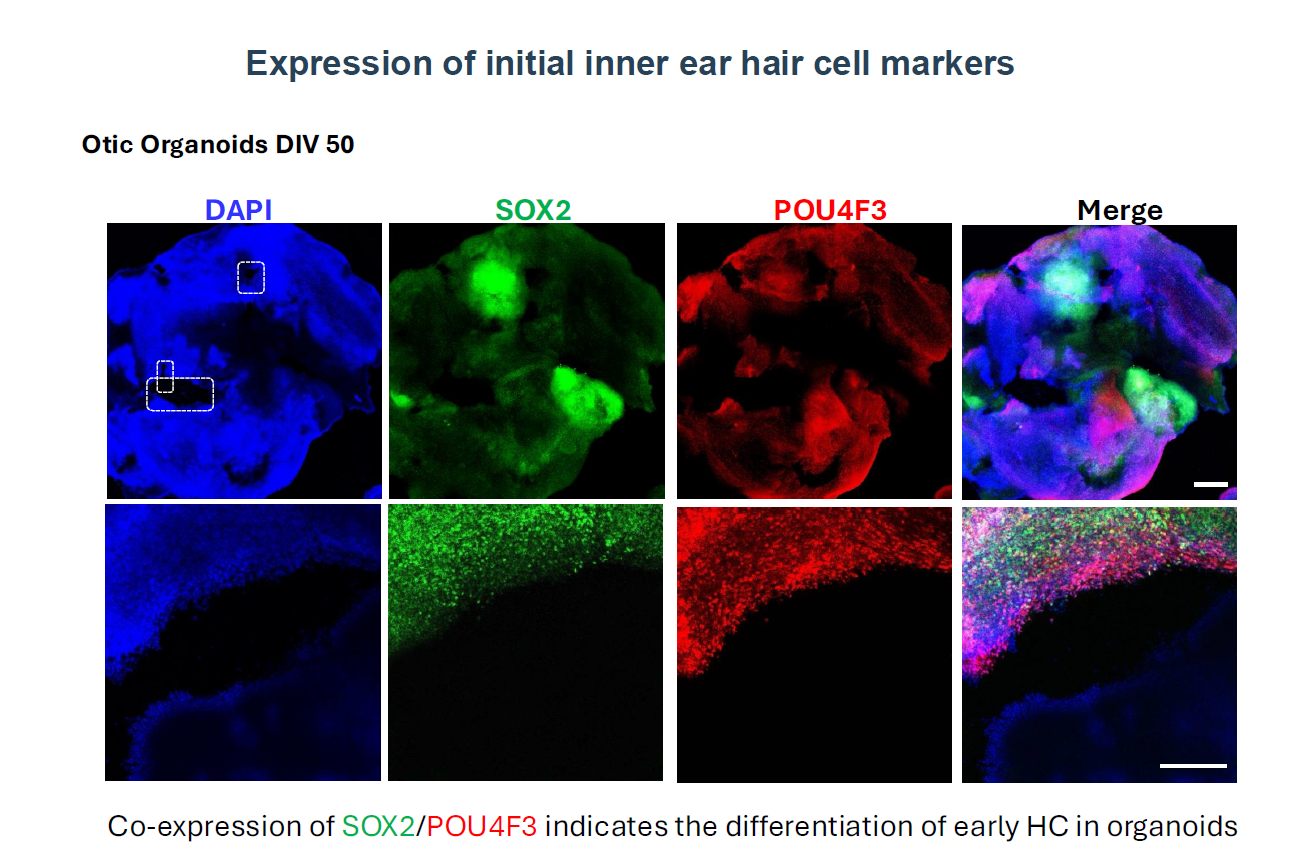
Pluripotent Stem Cell Derived-Human Inner Ear Organoids
We have first devised a new 2D-in vitro guidance protocol for human induced pluripotent stem cells (hiPSC) towards bona fide otic progenitor cells (hOPC) and then initial sensory hair cells (HC) (Lahlou et al., 2018). These results provided the proof of principles of early germ non-neuronal ectoderm layer formation and otic neurosensory induction in vitro. The aim of this project is to build up approaches to determine the chemical and biophysical environments to recapitulate the formation of functional human inner ear neurosensory epithelia from hiPSC and hOPC using a next-generation 3D-organoid strategy. Then, these cochlear organoids from the human inner ear will be used to study ototoxicity and neurosensory regeneration.
Project 2
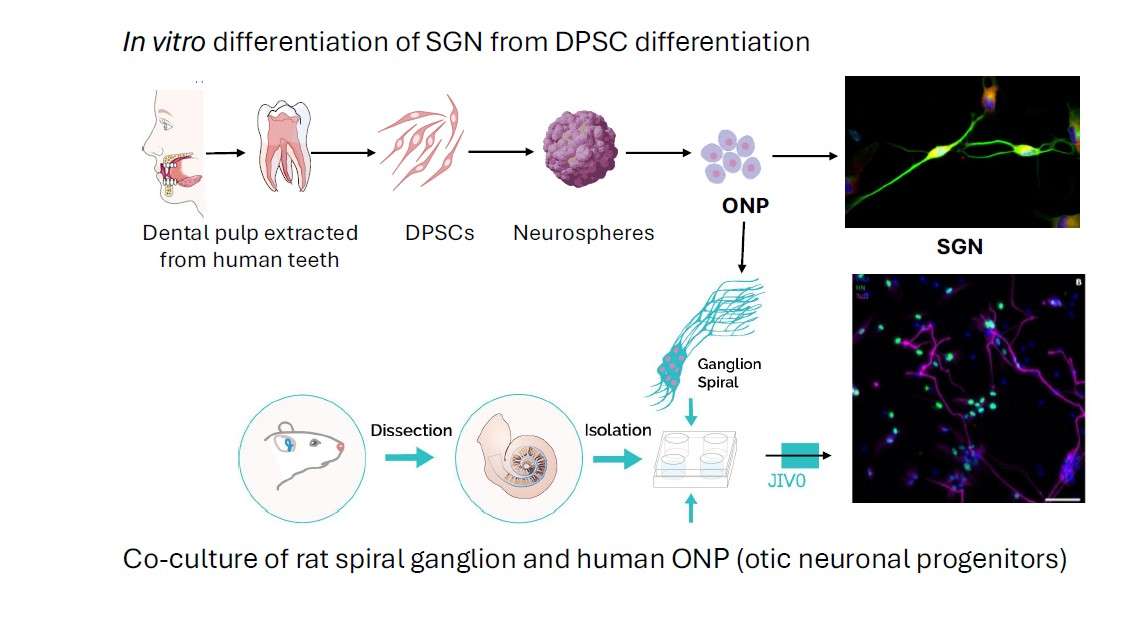
Engineering Otic Neurons from Dental Pulp Stem Cells for Auditory Neuropathy
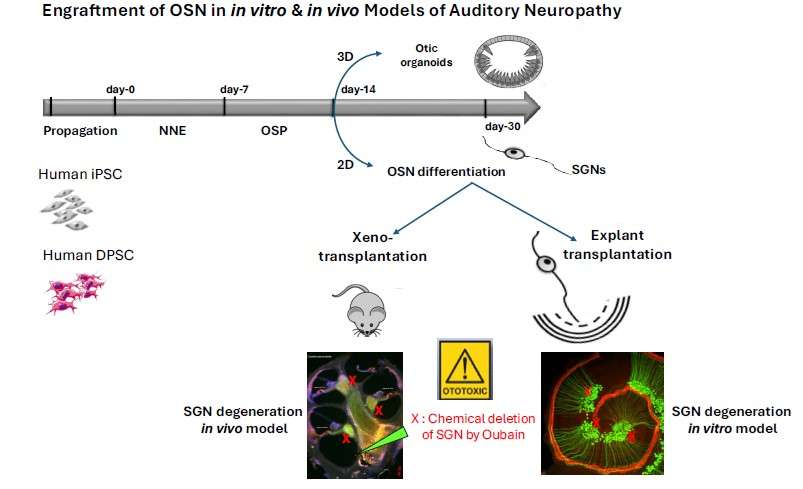
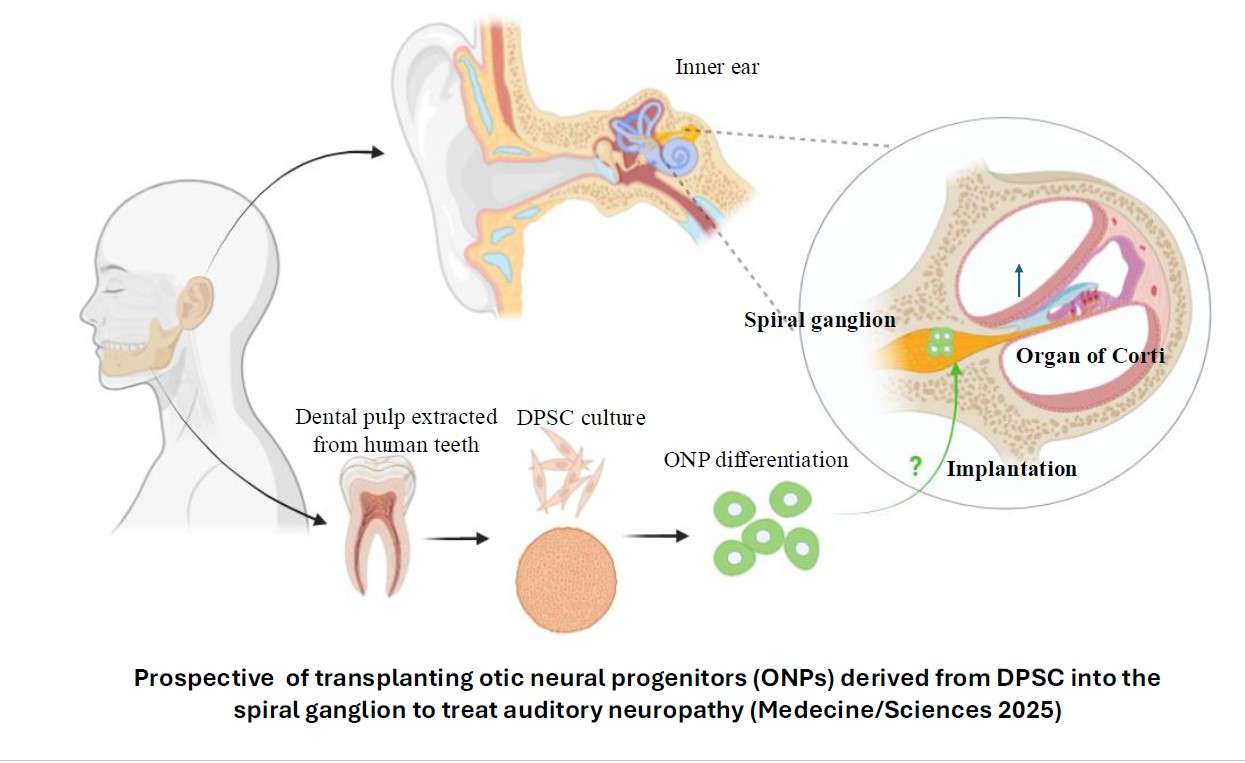
Auditory neuropathy is defined as a major neurosensory deficit due to the absence, or more often, dysfunction, of primary auditory neurons, bipolar neurons that make up the spiral ganglion of the inner ear. A cell therapy that could replace or repair the damaged auditory nerve is a potentially promising strategy for restoring auditory neuropathy. However, the prerequisite is the in vitro generation of otic neuronal progenitors from an easily accessible autologous source. Human dental pulp stem cells (hDPSC) are promising candidates for this type of treatment because they (1) have migratory properties, (2) exhibit plasticity and in vitro differentiation into multiple cell types. They represent (3) a highly accessible source of stem cells, particularly in the germs of wisdom teeth, which are extracted daily for orthodontic reasons and discarded as surgical waste in dental practices. The hDPSC can be used for autologous transplantation strategies in the auditory nerve. Our first objective would be to establish a reliable stepwise guidance in vitro procedure to produce a large amount of otic neurosensory progenitors from hDPSC differentiation. The second objective would then be to explore their potential for in vitro differentiation into auditory neurons and re-innervation of sensory HC in cell co-culture and in vivo models of auditory neuropathy.
Project 3
Transplantation Otic Cell Progenitors in Models of Auditory Neuropathy
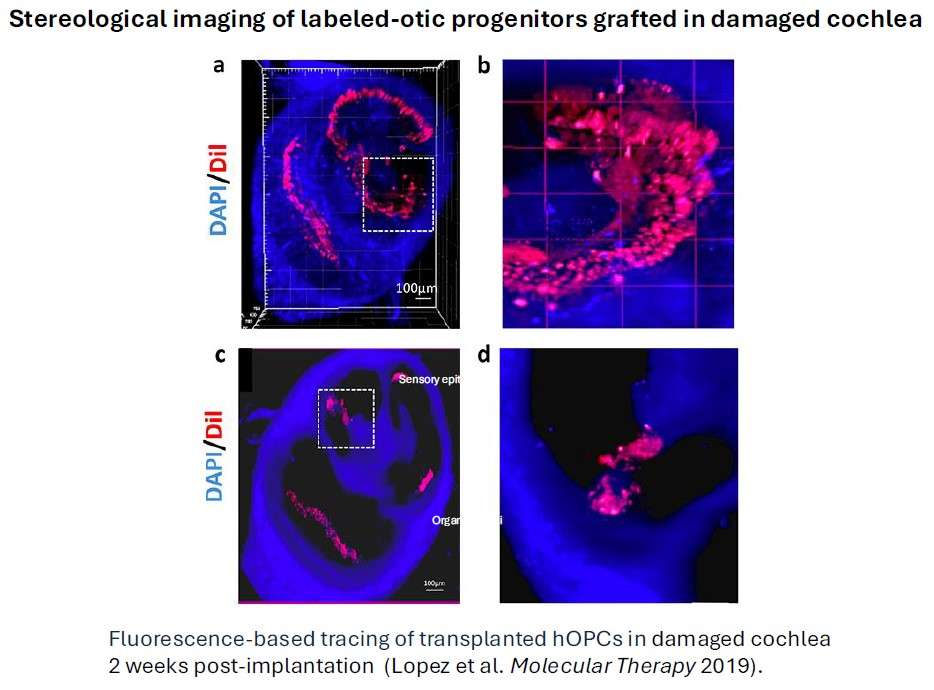
To test the regeneration of human mesenchymal DPSC and iPSC-derived otic neurosensory progenitors (OSP) in in vitro and in vivo models of auditory neuropathy. We use first an in vitro co-culture model with denervated cochlear organotypic explants to test the potential of OSP neuronal reconnection and synaptogenesis with native SGN and HC. Then, to determine whether we could replace neurons lost due to primary neuronal degeneration, we will engraft either iPSC or DPSC-derived OSP into the cochlear nerve trunk in immunosuppressed mouse after destroying the cochlear nerve (spiral ganglion) cells while leaving HC intact by ouabain exposure.
We have previously provided the proof of principle that human otic progenitors derived from hiPSC were able to survive and partially differentiate upon transplantation into a Guinea pig model of ototoxicity.
Collaborations:
- Albert Edge (Harvard Medical School)
- John Devos (IRMB, CHU, Montpellier)
- Christian Chabbert (CNRS, AMU Marseille)
- Pierre Gaudriault (Cherry Biotech, Rennes)
- Annelies Schrott-Fischer (Medical University of Innsbruck, Austria)
Article Covers
The press talks about us
Audiology infos N39 2015Audiology infos N41 2015Figaro 2022ISSCR 2017Observatoire Groupe Optic 200
Fundings:
Recent publications:
Messat Y, Dutilleul PC, Zine A (2025).Teeth at the bedside of the earMed Sci , 41, 628-630.
Messat Y, Martin-Fernandez M, Assou S, Chung K, Guérin F, Gergely C, Cuisinier F, Zine A. (2024). Differentiation of spiral ganglion neurons from human dental pulp stem cells: a further step towards autologous auditory nerve recovery. Int J Mol Sci, 25(16):9115.
Chung K, Millet M, Rouillon L, Zine A (2024). Timing and graded BMP signalling determines fate of neural crest and ectodermal placode derivatives from pluripotent stem cells. Biomedicines, 12, 2262.
Zine A, Fritzsch B (2023). Early steps towards hearing: placodes and sensory development. Int. J. Mol. Sci, 24(8), 6994; https://doi.org/10.3390/ijms24086994.
Elliott K, Fritzsch B, Yamoah Ebenezer, Zine A. (2022). Age-related hearing loss: sensory and neural etiology and their interdependence. Front Aging Neurosci 14:814528.
Zine A, Messat Y, Fritzsch B (2021). A human induced pluripotent stem cell-based modular platform to challenge sensorineural hearing loss.Stem Cells. doi: 10.1002/stem.3346.
JJohnson-Chacko L, Lahlou H, Steinacher C, Assou S, Messat Y, Dudas J, Edge A, Crespo B, Crosier M, Sergi C, Schrott-Fischer A, Zine A (2021). Transcriptome-wide analysis reveals a role for extracellular matrix and integrin receptor genes in otic neurosensory differentiation from human IPSCs. Int. J. Mol. Sci. 22,10849. https://doi.org/ 10.3390/Ijms221910849.
Kojima K, Nishida AT, Tashiro K, Hirota K, Nishio T, Murata M, Kato N, Kawaguchi S, Zine A, Ito J, Van De Water TR (2020). Isolation and characterization of mammalian otic progenitor cells that can differentiate into both sensory epithelial and neuronal cell lineages.Anat Rec303(3):451-460.
Lopez A, Lahlou H, Cazals Y, Brezun JM, Ripoll C, Quan W, Edge A, Zine A (2019). Engraftment of human stem cell-derived otic progenitors in the damaged cochlea.Molecular Therapy27: 1101-1113.
Lahlou H, Nivet E, Lopez A, Fontbonne A, Assou S, Zine A (2018). Enrichment and characterization of human otic sensory progenitors derived from induced pluripotent stem cells (2018).Front Mol Neurosci11:452.
Lahlou, H, Lopez Juarez, A, Fontbonne, A, Nivet, E, Zine A (2018). Modeling human early otic sensory cell development with induced pluripotent stem cellsPLoS One13(6): e0198954.
Abboud N, Fontbonne A, Watabe I, Tonetto A, Brezun JM, Feron F, Zine A (2017). Culture conditions impact the maturation of traceable, transplantable mouse embryonic stem cell-derived otic progenitor cells.JTissue Eng Regen Med11:2629-2642.
Dinh C, Goncalves S, Bas E, Van De Water T, Zine A (2015). Molecular regulation of auditory hair cell death and approaches to protect sensory receptor cells and/or stimulate repair following acoustic trauma.Front Cell Neurosci9:96.
Zine A (2014). 3D revolution of stem cells: Generation of ear sensory epithelia in vitro.Med Sci. 30: 952-954.
Zine A, Lowenheim, H, Fritzsch B (2014).Toward translating molecular ear development to generate hair cells from stem cells. In: Kursad T, eds.Adult stem cells. Springer111-161.